A) 0.75 atm
B) 0.55 atm
C) 0.68 atm
D) 0.85 atm
Correct Answer: A
Solution :
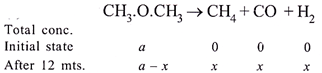 |
| Calculation of initial concentration \[(a)\,\] of\[C{{H}_{3}}OC{{H}_{3}}\] |
| \[PV=nRT\]or \[\frac{n}{V}=\frac{P}{RT}\] |
| \[=\frac{P}{RT}=\frac{0.4}{0.082\times 773}=6.31\times {{10}^{-3}}mol\,{{L}^{-1}}\] |
| calculation of k |
| \[k=\frac{0.693}{{{t}_{{}^{1}/{}_{2}}}}=\frac{0.693}{14.5}=4.78\times {{10}^{-2}}{{\min }^{-1}}\] |
| Substituting the values in the first order equation. |
| \[k=\frac{2.303}{t}\log \frac{a}{a-x}\] |
| \[4.78\times {{10}^{-2}}=\frac{2.303}{12}\log \frac{a}{a-x}\] |
| \[\frac{a}{a-x}=1.77446\] |
| \[a-x=\frac{6.31\times 1{{0}^{-3}}}{1.77446}mol\,{{L}^{-1}}\] |
| \[=3.556\times 1{{0}^{-3}}mol\,{{L}^{-1}}\] |
| \[\therefore \]\[x=(6.310-3.556)\times {{10}^{-3}}mol\,{{L}^{-1}}\] |
| \[=2.754\times {{10}^{-3}}mol\,{{L}^{-1}}\] |
| \[\therefore \] After 12 min. Total no. of \[mol\,{{L}^{-1}}\] |
| \[=a+2x=6.31\times {{10}^{-3}}+2\times 2.754\times {{10}^{-3}}\] |
| \[=11.818\times {{10}^{-3}}\] |
| \[\therefore \]\[P=\frac{n}{V}RT=11.818\times {{10}^{-3}}\times 0.082\times 773\] |
| \[=0.75atm\] |
You need to login to perform this action.
You will be redirected in
3 sec
