A)
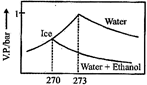
B)
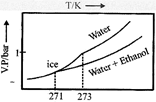
C)
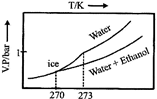
D)
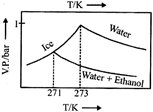
Correct Answer: C
Solution :
| As T increase, V.P. increase \[\Delta {{T}_{f}}={{K}_{f}}\times m\] (m=molality of solute) |
| \[273-{{T}_{f}}'=2\times \frac{34.5\times 1000}{46\times 500}\] |
| \[\therefore \] \[{{T}_{f}}'=270K\] |
| Thus freezing point of solution = 270K. Further as T increases, vapour pressure increases. Hence these coincide with the curve gives in [c]. |
You need to login to perform this action.
You will be redirected in
3 sec
