| Assuming the formation of an ideal solution, determine the boiling point of a mixture containing 1560 g benzene (molar mass = 78) and 1125 g chlorobenzene (molar mass = 112.54) using the following against an external pressure of 1000 Torr. |
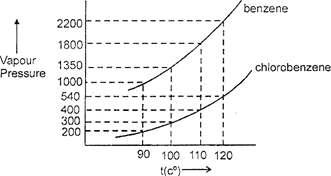 |
A) \[90{}^\circ C\]
B) \[100{}^\circ C\]
C) \[110{}^\circ \]
D) \[120{}^\circ C\]
Correct Answer: B
Solution :
| \[20\,\,\text{mole}\,{{C}_{6}}{{H}_{6}},\]\[10\,\,\text{mole}\,{{C}_{6}}{{H}_{5}}Cl\] \[\Rightarrow \]\[{{X}_{B}}=\frac{2}{3},\] \[{{X}_{C}}=\frac{1}{3}\] |
| at \[t=100{}^\circ C\] \[\Rightarrow \]\[{{p}_{s}}=300\times \frac{1}{3}+1350\times \frac{2}{3}=100\]\[+900\,(=1000)\] |
You need to login to perform this action.
You will be redirected in
3 sec
