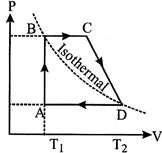
| Four moles of an ideal gas undergoes the cyclic process ABCDA as shown in figure. If \[3{{T}_{c}}=4{{T}_{B}}=12{{T}_{A}}=2400K,\] determine the work done by, the gas during the entire process. |
 |
A) 31.04 kJ
B) 13.04 kJ
C) 41.03 kJ
D) 14.03 kJ
Correct Answer: A
Solution :
| Let \[{{\operatorname{V}}_{A}},{{V}_{\operatorname{C}}}\], and \[{{\operatorname{V}}_{\operatorname{D}}}\]be respective volumes of the gas in the states A, C and D. Further, if \[{{P}_{A}}\] and \[{{P}_{B}}\] be the respective pressures of the gas in state A (or D) and B (or C), then, the work done by the gas, during the complete cycle is given as the area of the cycle on the P-V diagram. | |
| \[\therefore \]\[\Delta W\]= area of the trapezium ABCD | |
| \[=\left( {{P}_{B}}-{{P}_{A}} \right)\left[ {{V}_{D}}-{{V}_{A}})+\left( {{V}_{C}}-{{V}_{B}} \right) \right]\] | |
| \[=\left( {{P}_{B}}-{{P}_{A}} \right)\left[ {{V}_{C}}+{{V}_{D}}-2{{V}_{A}} \right]\] | ...(i) |
| For, the isochoric process \[\operatorname{A} \to B,\] | |
| We have \[\Delta P=\frac{nR}{V}\Delta T \] | |
| i-e., \[{{P}_{B}}-{{P}_{A}}\frac{nR}{{{V}_{A}}}[{{T}_{B}}-{{T}_{A}}]\] | ...(ii) |
| Further, for the process \[\operatorname{B}\to C\](which is isobaric) | |
| \[\operatorname{V}\propto \operatorname{T}\,\,\,\,i.e.,\frac{{{V}_{C}}}{{{V}_{B}}}=\frac{{{\operatorname{T}}_{\operatorname{C}}}}{{{\operatorname{T}}_{B}}}\] |
| Or \[{{V}_{C}}=\frac{{{T}_{C}}}{{{T}_{B}}}{{V}_{A}}\] |
| \[\left[ \because \,{{V}_{B}}-{{V}_{A}} \right]\] |
| For the process \[\operatorname{D} \to A \] (which is isobaric) |
| \[\operatorname{V}\propto \operatorname{T}\,\,\,i.e.,\,\frac{{{V}_{D}}}{{{V}_{A}}}=\frac{{{T}_{C}}}{{{T}_{A}}}...(iv)\] |
| [\[\because \]B and D lie on the same isotherm] |
| Work done |
| \[\Delta \operatorname{W}=\frac{\operatorname{nR}\left( {{\operatorname{T}}_{B}}-{{T}_{A}} \right)}{{{\operatorname{V}}_{A}}}\left[ \frac{{{T}_{B}}{{V}_{A}}}{{{T}_{A}}}+\frac{{{T}_{C}}{{T}_{A}}}{{{T}_{B}}}-2{{V}_{A}} \right]\]\[\Delta \operatorname{W}=\operatorname{nR}\left( {{\operatorname{T}}_{B}}-{{\operatorname{T}}_{A}} \right)\left[ \frac{{{T}_{B}}}{{{T}_{A}}}+\frac{{{T}_{C}}}{{{T}_{B}}}-2 \right]\]\[\Delta \operatorname{W}=\left( 4mol \right)\left( 8.314\frac{J}{mol-K} \right)\left( 600K-200K \right)\] |
| \[\left[ \frac{600K}{200K}+\frac{800K}{600K}-2 \right]=31.04kJ\] |
You need to login to perform this action.
You will be redirected in
3 sec
