| Consider the given plot of enthalpy of the following reaction between\[A\]and\[B.A+B\to C+D.\] Identify the incorrect statement, |
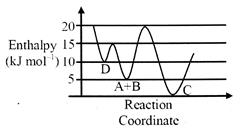 |
A) C is the thermodynamically stable product
B) Formation of A and B form C has highest enthalpy of activation.
C) Activation enthalpy to form C is\[5\,kJ\,mo{{l}^{-1}}\]
D) D is kinetically stable product.
Correct Answer: C
Solution :
| \[{{E}_{a}}=(d\to c)=15-0=15\] |
| \[{{E}_{a}}=(A+B)\to C=15\] |
| \[{{E}_{a}}=(A+B\to D)=10\] |
| \[{{E}_{a}}=C\to (A+B)=20.\] |
You need to login to perform this action.
You will be redirected in
3 sec
