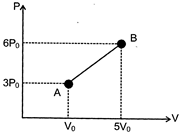
| One mole of a monoatomic ideal gas undergoes the process\[A\to B\] in the given P-V diagram. The specific heat for this process is: |
 |
A) \[\frac{3R}{2}\]
B) \[\frac{13R}{6}\]
C) \[\frac{5R}{2}\]
D) 2R
Correct Answer: B
Solution :
| Specific heat |
| \[C=\frac{\Delta Q}{\Delta T}=\frac{1}{\Delta T}\left( \Delta U+W \right)={{C}_{V}}+\frac{W}{\Delta T}\] |
| For the given process \[W=4{{V}_{0}}\frac{9{{P}_{0}}}{2}=18{{P}_{0}}{{V}_{0}}\] |
| Also,\[\Delta T={{T}_{2}}-{{T}_{1}}=\frac{\left( 6{{P}_{0}} \right)\left( 5{{V}_{0}} \right)}{R}-\frac{\left( 3{{P}_{0}} \right)}{R}=\frac{27{{P}_{0}}{{V}_{0}}}{R}\] |
| and \[{{C}_{V}}=\frac{3}{2}R;\]\[\therefore \,\,\,\,\,\,C=\frac{3R}{2}+\frac{2R}{3}=\frac{13R}{6}\] |
You need to login to perform this action.
You will be redirected in
3 sec
