A)

B)
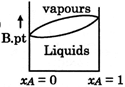
C)
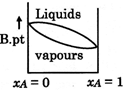
D)
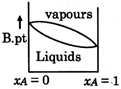
Correct Answer: D
Solution :
[D] \[\to \]Liquid which is more volatile have less boiling point \[\to \]At higher temperature vapour phase exist in place of liquid
\[\to \]Liquid which is more volatile have less boiling point \[\to \]At higher temperature vapour phase exist in place of liquid
You need to login to perform this action.
You will be redirected in
3 sec
