A)
B)
C)
D)
Correct Answer: B
Solution :
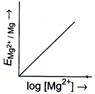
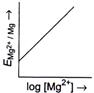
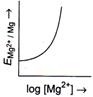
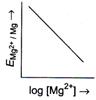
[b] \[{{E}_{M{{g}^{2+}}/Mg}}=E{{{}^\circ }_{M{{g}^{2+}}/Mg}}-\frac{0.059}{2}\log \frac{1}{M{{g}^{2+}}}\] or \[{{E}_{M{{g}^{2+}}/Mg}}=E{{{}^\circ }_{M{{g}^{2+}}/Mg}}+\frac{0.059}{2}\log \,M{{g}^{2+}}\] The given equation is of \[y=mx+c\]type, which is equation of straight line. So, graph of \[{{E}_{M{{g}^{2+}}|Mg}}(y)\,vs\,\log \,[M{{g}^{2+}}](x)\] is a straight line with slope \[\frac{0.059}{2}\]and intercept \[E{{{}^\circ }_{M{{g}^{2+}}|Mg}}.\]You need to login to perform this action.
You will be redirected in
3 sec
