A) 1/n appears as the intercept
B) Only 1/n appears as the slope
C) \[\log \left( \frac{1}{n} \right)\]appears as the intercept
D) [d] Both k and 1/n appear in the slope term
Correct Answer: B
Solution :
| [b] According to Freundlich adsorption isotherm, \[\frac{x}{m}=k{{p}^{1/n}}\] |
| On taking logarithm of both sides, we get \[\log \frac{x}{m}=\log k+\log {{p}^{1/n}}\] or \[\log \frac{x}{m}=\log k+\frac{1}{n}\log p\] |
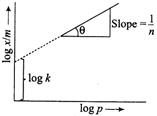 |
| On compairing the above equation, with equation of straight line, i.e. |
| \[y=c+mx,\] we get \[y=\log \frac{x}{m},\] |
| c = intercept = log k |
| m = slope \[=\frac{1}{n}\] and \[=x=\log p\] |
| Thus, option [b] is correct. |
You need to login to perform this action.
You will be redirected in
3 sec
