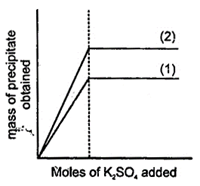
A) \[BaS{{O}_{4}}\]and\[MgS{{O}_{4}}\], are precipitated in (1) and (2)
B) \[BaS{{O}_{4}}\] and \[KCl{{O}_{4}}\], are precipitated in (1) and (2)
C) \[KCl{{O}_{4}}\] and \[KCl\] are precipitated In (1) and (2)
D) \[KCl\] and \[MgS{{O}_{4}}\]. are precipitated in (1) and (2)
Correct Answer: B
You need to login to perform this action.
You will be redirected in
3 sec
