A) 1
B) \[\frac{1}{2}\]
C) \[\frac{1}{4}\]
D) 2
Correct Answer: D
Solution :
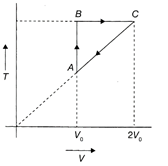
CA is an isobaric process and \[{{V}_{C}}=2{{V}_{A}}\] \[\Rightarrow \]\[{{T}_{C}}=2{{T}_{A}}.{{T}_{B}}={{T}_{C}}=2{{T}_{A}}\] AB is an isochoric process with \[{{T}_{B}}=2{{T}_{A}}.\] \[\therefore \]\[{{P}_{B}}=2{{P}_{A}}\]or \[{{P}_{B}}/{{P}_{A}}=2\] Hence, the correction option is (d).You need to login to perform this action.
You will be redirected in
3 sec
