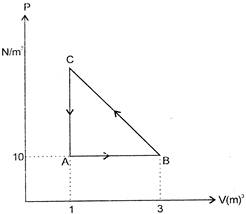
A) \[-10J\]
B) \[-30J\]
C) \[-15J\]
D) \[20J\]
Correct Answer: A
Solution :
Heat involved in the process \[\Delta Q={{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}+\Delta U,\] (\[\Delta U=0\] for the complete cycle) \[\Rightarrow \] \[10\times (3-1)+{{W}_{BC}}+0=10J\] \[{{W}_{BC}}=-10J\]You need to login to perform this action.
You will be redirected in
3 sec
