A) \[-5\,J\]
B) \[-20\,J\]
C) \[-15\,J\]
D) \[-25\,J\]
Correct Answer: A
Solution :
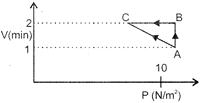
Key Idea: Process is cyclic, so change in internal energy is zero. We know that work done by gas is given by \[W=P\Delta V\] Where P is pressure and \[\Delta V\] the change in volume. Total work done by gas is: \[W={{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}\] ?..(i) According to first law of thermodynamics \[\Delta Q=\Delta U+W\] .....(ii) Where \[\Delta Q\]is heat supplied, \[\Delta V\] the change in internal energy which is zero and W the work done. \[\therefore \] \[{{W}_{AB}}=P\Delta V=10(2-1)=10J.\] \[{{W}_{BC}}=P\Delta V\] \[=2\times 0=0.\] Thus, equations (i) and (ii) will be written as \[5=0+(10+0+{{W}_{CA}}).\] \[\Rightarrow \] \[{{W}_{CA}}=-5J.\]
\[W={{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}\] ?..(i) According to first law of thermodynamics \[\Delta Q=\Delta U+W\] .....(ii) Where \[\Delta Q\]is heat supplied, \[\Delta V\] the change in internal energy which is zero and W the work done. \[\therefore \] \[{{W}_{AB}}=P\Delta V=10(2-1)=10J.\] \[{{W}_{BC}}=P\Delta V\] \[=2\times 0=0.\] Thus, equations (i) and (ii) will be written as \[5=0+(10+0+{{W}_{CA}}).\] \[\Rightarrow \] \[{{W}_{CA}}=-5J.\]
You need to login to perform this action.
You will be redirected in
3 sec
