A) ONF is isoelectronic with \[{{O}_{2}}{{N}^{-}}\]
B) \[O{{F}_{2}}\]is an oxide of fluorine
C) \[C{{l}_{2}}{{O}_{7}}\]is an. anhydride of perchloric acid
D) \[{{O}_{3}}\] molecule is bent
Correct Answer: B
Solution :
(i) No. of electron in ONF = 24 No. of electron in \[NO_{2}^{-}=24\] Both are isoelectronic (ii)\[O{{F}_{2}}\] is a fluoride of oxygen and not oxide of fluorine because EN of fluorine is more than oxygen \[O{{F}_{2}}=\]oxygen difluoride (iii) \[C{{l}_{2}}{{O}_{7}}\]is an anhydride of perchloric acid \[2HCl{{O}_{4}}\xrightarrow[-{{H}_{2}}O]{\Delta }C{{l}_{2}}{{O}_{7}}\] (iv)\[{{O}_{3}}\]molecule is bent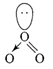 shape
shape
You need to login to perform this action.
You will be redirected in
3 sec
