A) \[N{{H}_{3}},P{{H}_{3}}\]
B) \[Xe{{F}_{4}},Xe{{O}_{4}}\]
C) \[SiC{{l}_{4}},PC{{l}_{4}}\]
D) Diamond, silicon carbide
Correct Answer: B
Solution :
Hybridization of \[N{{H}_{3}}[\sigma =3,lp=1]\] \[s{{p}^{3}}\]geometry: tetrahedral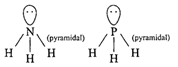 (ii) Structure of\[Xe{{F}_{4}}\]is square planar.
(ii) Structure of\[Xe{{F}_{4}}\]is square planar. 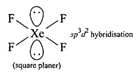 Structure of\[Xe{{O}_{4}}\]is tetrahedral
Structure of\[Xe{{O}_{4}}\]is tetrahedral 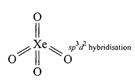 So \[Xe{{F}_{4}}\]and \[Xe{{O}_{4}}\]are not isostructural (iii) Structure of \[SiC{{l}_{4}}\]is tetrahedral
So \[Xe{{F}_{4}}\]and \[Xe{{O}_{4}}\]are not isostructural (iii) Structure of \[SiC{{l}_{4}}\]is tetrahedral 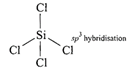 Structure of \[PCl_{4}^{+}\]is tetrahedral
Structure of \[PCl_{4}^{+}\]is tetrahedral 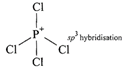 (iv) Both diamond and SiC are isostructural because both have tetrahedral arrangement and central atom is \[s{{p}^{3}}\]hybridised.
(iv) Both diamond and SiC are isostructural because both have tetrahedral arrangement and central atom is \[s{{p}^{3}}\]hybridised.
You need to login to perform this action.
You will be redirected in
3 sec
