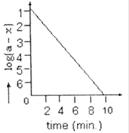
A) \[10\,{{s}^{-1}}\]
B) \[{{10}^{-2}}\,{{s}^{-1}}\]
C) \[{{10}^{-3}}\,{{s}^{-1}}\]
D) None of these
Correct Answer: A
Solution :
For a first order reaction slope = \[-\frac{k}{2.303}\] or \[\frac{-6}{60\times 10}\,\,=\,\,-\frac{k}{2.303}\,\,=\,\,{{10}^{-2}}\,{{s}^{-1}}\]You need to login to perform this action.
You will be redirected in
3 sec
