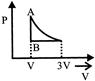
A)
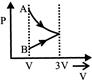
B)
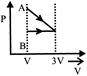
C)
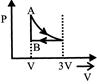
D)
Correct Answer: A
Solution :
[a]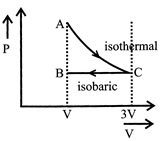 AC = Isothermal process CB = Pressure constant Isobaric
AC = Isothermal process CB = Pressure constant Isobaric
You need to login to perform this action.
You will be redirected in
3 sec
