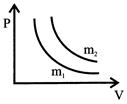
A) \[{{m}_{1}}>{{m}_{2}}\]
B) \[{{m}_{1}}={{m}_{2}}\]
C) \[{{m}_{1}}<{{m}_{2}}\]
D) \[{{m}_{1}}>{{m}_{2}}+2\]
Correct Answer: C
Solution :
[c] \[PV=\frac{m}{M}RT\] \[For\text{ }{{1}^{st}}\text{ }graph,P=\frac{{{m}_{1}}}{M}\frac{RT}{{{V}_{1}}}~~~....\left( 1 \right)\] \[For\text{ }{{\text{2}}^{nd}}\text{ }graph,P=\frac{{{m}_{2}}}{M}\frac{RT}{{{V}_{2}}}~~~....\left( 2 \right)\] From (1) and (2) \[\frac{{{m}_{1}}}{{{m}_{2}}}=\frac{{{V}_{1}}}{{{V}_{2}}}<1~{{V}_{2}}>{{V}_{1}}\] \[{{m}_{1}}<{{m}_{2}}\]
\[PV=\frac{m}{M}RT\] \[For\text{ }{{1}^{st}}\text{ }graph,P=\frac{{{m}_{1}}}{M}\frac{RT}{{{V}_{1}}}~~~....\left( 1 \right)\] \[For\text{ }{{\text{2}}^{nd}}\text{ }graph,P=\frac{{{m}_{2}}}{M}\frac{RT}{{{V}_{2}}}~~~....\left( 2 \right)\] From (1) and (2) \[\frac{{{m}_{1}}}{{{m}_{2}}}=\frac{{{V}_{1}}}{{{V}_{2}}}<1~{{V}_{2}}>{{V}_{1}}\] \[{{m}_{1}}<{{m}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
