 The reaction must be:
The reaction must be:
A) Exothermic
B) Endothermic
C) One with negligible enthalpy change
D) Highly spontaneous at ordinary temperature
Correct Answer: A
Solution :
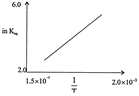
[a] Exothermic According to van't Hoff equation, giving the effect of temperature on equilibrium constant, \[{{k}_{eq}}=A{{e}^{-\Delta H/RT}}\] or In \[{{k}_{eq}}=In\,A\,-\frac{\Delta \,H}{RT}\] In the given plot, InK increases with increase of 1/T i.e slope of the plot is +ve. Hence AH must be -ve i.e. reaction is exothermic.You need to login to perform this action.
You will be redirected in
3 sec
