A) One
B) Two
C) Six
D) Three
Correct Answer: A
Solution :
[a] One Since EDTA is hex dentate, therefore, one molecule of EDTA combines with \[C{{a}^{2+}}\] ion to form an octahedral complex.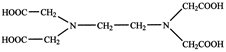
You need to login to perform this action.
You will be redirected in
3 sec
