A) \[Xe{{F}_{4}}\]
B) \[BF_{4}^{-}\]
C) \[SF_{4}^{{}}\]
D) \[SiF_{4}^{{}}\]
Correct Answer: C
Solution :
[c] \[S{{F}_{4}}\] \[Xe{{F}_{4}}\]is squar planar while \[BF_{4}^{-}\]and are\[Si{{F}_{4}}\] tetrahedral, all M-F bonds are equal in these molecules. However, in \[S{{F}_{4}}\] there is \[s{{p}^{3}}\]d-hybridisation having two axial and two equatorial S-F bonds. Thus, all the bonds in \[S{{F}_{4}}\] are not equal.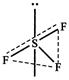 Lone pair occupying axial position.
Lone pair occupying axial position.
You need to login to perform this action.
You will be redirected in
3 sec
