A) \[\frac{1}{4\pi {{\varepsilon }_{0}}}\,\,\frac{4{{e}^{2}}}{3{{a}^{2}}}\]
B) \[\frac{1}{4\pi {{\varepsilon }_{0}}}\,\,\frac{16{{e}^{2}}}{3{{a}^{2}}}\]
C) \[\frac{1}{4\pi {{\varepsilon }_{0}}}\,\,\frac{32{{e}^{2}}}{3{{a}^{2}}}\]
D) zero
Correct Answer: D
Solution :
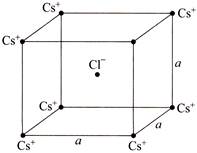
The given crystal structure is a body centered cubic structure. The electrostatic force, \[F=\frac{1}{4\pi {{\varepsilon }_{0}}}\,\,\frac{{{q}_{1}}{{q}_{2}}}{{{r}^{2}}}\] Since one \[{{\operatorname{Cs}}^{+}}\] ion is balanced by diagonally opposite \[{{\operatorname{Cs}}^{+}}\] ion, net electrostatic force on \[C{{l}^{-}}\] ion due to eight \[{{\operatorname{Cs}}^{+}}\] ions is zero.You need to login to perform this action.
You will be redirected in
3 sec
