A) \[15\,%\]
B) \[50\,%\]
C) \[20\,%\]
D) \[20\,%\]
Correct Answer: B
Solution :
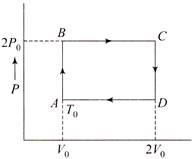
Maximum efficiency of reversible process is given by \[\eta =1-\frac{T}{T'}\] At \[{{\operatorname{AP}}_{0}}{{V}_{0}}\,\,=\,\,nR{{T}_{0}}\] At \[\operatorname{B}2{{P}_{0}}{{V}_{0}}=nRT'\] \[T'\,\,=\,\,2{{T}_{0}}\] \[\therefore \,\,\,\,\,\,\,\eta =1-\frac{{{T}_{0}}}{2{{T}_{0}}}=\frac{1}{2}=50\,%\]You need to login to perform this action.
You will be redirected in
3 sec
