A)
![]()
B)
![]()
C)
![]()
D) Both [a] and [c]
Correct Answer: D
Solution :
In both two cases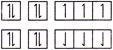 Hund?s rule of maximum multiplicity is follow.
Hund?s rule of maximum multiplicity is follow.
You need to login to perform this action.
You will be redirected in
3 sec
