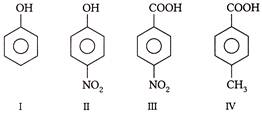
A) \[I<II<IU<IV\]
B) \[I<II <IV<III\]
C) \[I>II>IV<III\]
D) \[I<II>III<IV\]
Correct Answer: B
Solution :
Presence of electron with drawing group on ring or aromatic nucleus increases its acidic strength decreasing electron density.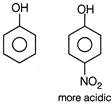 On the other hand, electron donating group. Present on aromatic nucleus (here) decreases acidic strength due to increase in acidic strength around nucleus.
On the other hand, electron donating group. Present on aromatic nucleus (here) decreases acidic strength due to increase in acidic strength around nucleus. 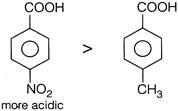 Also we know that carboxylic acid is more acidic than phenol due to more stable conjugate base of benzoic acid.
Also we know that carboxylic acid is more acidic than phenol due to more stable conjugate base of benzoic acid. 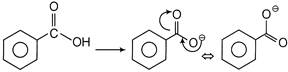 More stability of conjugate base is due to resonance.
More stability of conjugate base is due to resonance.
You need to login to perform this action.
You will be redirected in
3 sec
