A) \[\frac{1}{4}\]
B) \[\frac{1}{2}\]
C) 1
D) 4
Correct Answer: C
Solution :
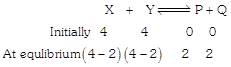 Equilibrium constant is\[{{K}_{c}}=\frac{[C][D]}{[A][B]}=1\]
Equilibrium constant is\[{{K}_{c}}=\frac{[C][D]}{[A][B]}=1\]
You need to login to perform this action.
You will be redirected in
3 sec
