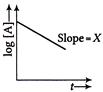
 X is equal to
X is equal to
A) \[\frac{0.693}{k}\]
B) \[\frac{k}{2.303}\]
C) \[-\frac{k}{2.303}\]
D) \[\log \,{{\left[ A \right]}_{0}}\]
Correct Answer: C
Solution :
From first order rate equation \[\log \,[A]=\frac{kt}{2.303}+\log {{[A]}_{0}}\] \[\operatorname{Y}=\,\,m x + c\] \[m=\frac{k}{2.303}\] \[Y\,\,=\,\,\log \,[A]\] \[X=t\] \[\operatorname{C}=\,\,\log {{\left[ A \right]}_{0}}\]You need to login to perform this action.
You will be redirected in
3 sec
