A) \[2{{p}_{i}}\left( \frac{{{T}_{2}}}{{{T}_{1}}+{{T}_{2}}} \right)\]
B) \[2{{p}_{i}}\left( \frac{{{T}_{1}}{{T}_{2}}}{{{T}_{1}}+{{T}_{2}}} \right)\]
C) \[{{p}_{i}}\left( \frac{{{T}_{1}}{{T}_{2}}}{{{T}_{1}}+{{T}_{2}}} \right)\]
D) \[2{{p}_{i}}\left( \frac{{{T}_{1}}}{{{T}_{1}}+{{T}_{2}}} \right)\]
Correct Answer: A
Solution :
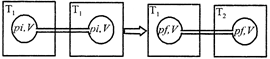
[a] For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant, i.e. \[V\propto n\] Hence in the given case. Initial moles and final moles are equal \[{{({{n}_{T}})}_{i}}={{({{n}_{T}})}_{f}}\] \[\frac{{{P}_{i}}V}{R{{T}_{1}}}+\frac{{{P}_{i}}V}{R{{T}_{1}}}=\frac{{{P}_{f}}V}{R{{T}_{1}}}+\frac{{{P}_{f}}V}{R{{T}_{2}}}\] \[2\frac{{{P}_{i}}}{{{T}_{1}}}=\frac{{{P}_{f}}}{{{T}_{1}}}+\frac{{{P}_{f}}}{{{T}_{2}}}\] \[{{P}_{f}}=\frac{2\,\,{{P}_{i}}{{T}_{2}}}{{{T}_{1}}+{{T}_{2}}}\]You need to login to perform this action.
You will be redirected in
3 sec
