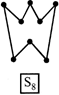
A) The S-S-S bond angles in the \[{{S}_{8}}\] and \[{{S}_{6}}\] rings are the same.
B) Rhombic and monoclinic sulphur have \[{{S}_{8}}\] molecules.
C) \[{{S}_{2}}\] is paramagnetic like oxygen
D) \[{{S}_{8}}\] ring has a crown shape.
Correct Answer: A
Solution :
[a] The \[{{S}_{6}}\] molecule has a chair-form hexagon ring with the same bond length as that in \[{{S}_{8}},\] but with some what smaller bond angles i.e. bond lengths are same but bond angles are different.
You need to login to perform this action.
You will be redirected in
3 sec
