A)
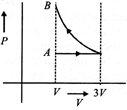
B)
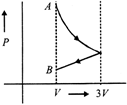
C)
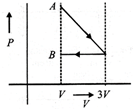
D)
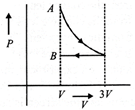
Correct Answer: D
Solution :
[d] 1st process is isothermal expansion which is only correct shown in option [d] 2nd process is isobaric compression which is correctly shown in option [d]You need to login to perform this action.
You will be redirected in
3 sec
