| Using Bohr's postulates for hydrogen atom, show that the total energy (E) of the electron in the stationary states can |
| be expressed as the sum of kinetic energy (K) and potential energy (U). Show that, \[K=-U/2.\] |
Answer:
The energy E of an electron in an orbit is the sum of kinetic and potential energies. Let us consider a nucleus has charge Ze and an electron e is moving it in a circular path of radius \[r.\]The electrostatic force on the electron, 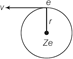 \[F=\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{Ze.e}{{{r}^{2}}}\] [According to Coulomb?s law] Since, electron is moving in a circular orbit. Centripetal force on the electron, \[F=m{{v}^{2}}\,/\,r.\] Where, \[m\]= mass of electron And \[v\]= speed of the electron. Hence, \[\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}{{r}^{2}}}=\frac{m{{v}^{2}}}{r}\] \[\therefore \] \[r=\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}m{{v}^{2}}}\] ??? (i) According to Bohr?s quantisation postulates, the angular momentum of the electron, in nth orbit, \[mvr=n.\frac{h}{2\pi }\] ???.. (ii) From Eqs. (i) and (ii), we get \[v=\frac{Z{{e}^{2}}}{2{{\varepsilon }_{0}}hn}\] ???. (iii) \[\therefore \] Kinetic energy \[=\frac{1}{2}\,m{{v}^{2}}=\frac{m{{Z}^{2}}{{e}^{4}}}{8\varepsilon _{0}^{2}{{h}^{2}}}\,\left( \frac{1}{{{n}^{2}}} \right)\] In terms of Rydberg constant R, its simplified form is \[KE=\frac{Rhc}{{{n}^{2}}}\] ?? (iv) \[\left[ \therefore \,\,R=\frac{m{{e}^{4}}}{8\varepsilon _{0}^{2}c{{h}^{3}}} \right]\] The potential energy of the electron in an orbit of radius \[r\] due to the electrostatic attraction by the nucleus is given by \[U=\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{(Ze)\,(-\,e)}{r}=-\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{Z{{e}^{2}}}{r}\] Putting the value of r from Eq. (i) and in terms of Rydberg constant R, its simplified form is \[U=-\frac{2Rhc}{{{n}^{2}}}\] ???.. (v) The total energy of the electron is therefore, \[E=KE+U\] \[=\frac{Z{{e}^{2}}}{8\pi {{\varepsilon }_{0}}r}-\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}r}=\frac{Z{{e}^{2}}}{8\pi {{\varepsilon }_{0}}r}\] Substituting for r from Eq. (i), we get \[E=-\frac{m{{Z}^{2}}{{e}^{4}}}{8{{\varepsilon }_{0}}^{2}{{h}^{2}}}\,\left( \frac{1}{{{n}^{2}}} \right)=\frac{-Rhc}{{{n}^{2}}}\] Where, n = 1, 2, 3,.... This is the expression for the energy of the electron in the nth orbit. For hydrogen atom \[Z=1,\] substituting the standard values, we get \[{{E}_{n}}=\frac{-13.6}{{{n}^{2}}}eV.\]Negative energy of the electron shows that the electron is bound to the nucleus and is not free to leave it. From Eqs. (iv) and (v), we get \[K=\frac{-\,U}{2}\]
\[F=\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{Ze.e}{{{r}^{2}}}\] [According to Coulomb?s law] Since, electron is moving in a circular orbit. Centripetal force on the electron, \[F=m{{v}^{2}}\,/\,r.\] Where, \[m\]= mass of electron And \[v\]= speed of the electron. Hence, \[\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}{{r}^{2}}}=\frac{m{{v}^{2}}}{r}\] \[\therefore \] \[r=\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}m{{v}^{2}}}\] ??? (i) According to Bohr?s quantisation postulates, the angular momentum of the electron, in nth orbit, \[mvr=n.\frac{h}{2\pi }\] ???.. (ii) From Eqs. (i) and (ii), we get \[v=\frac{Z{{e}^{2}}}{2{{\varepsilon }_{0}}hn}\] ???. (iii) \[\therefore \] Kinetic energy \[=\frac{1}{2}\,m{{v}^{2}}=\frac{m{{Z}^{2}}{{e}^{4}}}{8\varepsilon _{0}^{2}{{h}^{2}}}\,\left( \frac{1}{{{n}^{2}}} \right)\] In terms of Rydberg constant R, its simplified form is \[KE=\frac{Rhc}{{{n}^{2}}}\] ?? (iv) \[\left[ \therefore \,\,R=\frac{m{{e}^{4}}}{8\varepsilon _{0}^{2}c{{h}^{3}}} \right]\] The potential energy of the electron in an orbit of radius \[r\] due to the electrostatic attraction by the nucleus is given by \[U=\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{(Ze)\,(-\,e)}{r}=-\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{Z{{e}^{2}}}{r}\] Putting the value of r from Eq. (i) and in terms of Rydberg constant R, its simplified form is \[U=-\frac{2Rhc}{{{n}^{2}}}\] ???.. (v) The total energy of the electron is therefore, \[E=KE+U\] \[=\frac{Z{{e}^{2}}}{8\pi {{\varepsilon }_{0}}r}-\frac{Z{{e}^{2}}}{4\pi {{\varepsilon }_{0}}r}=\frac{Z{{e}^{2}}}{8\pi {{\varepsilon }_{0}}r}\] Substituting for r from Eq. (i), we get \[E=-\frac{m{{Z}^{2}}{{e}^{4}}}{8{{\varepsilon }_{0}}^{2}{{h}^{2}}}\,\left( \frac{1}{{{n}^{2}}} \right)=\frac{-Rhc}{{{n}^{2}}}\] Where, n = 1, 2, 3,.... This is the expression for the energy of the electron in the nth orbit. For hydrogen atom \[Z=1,\] substituting the standard values, we get \[{{E}_{n}}=\frac{-13.6}{{{n}^{2}}}eV.\]Negative energy of the electron shows that the electron is bound to the nucleus and is not free to leave it. From Eqs. (iv) and (v), we get \[K=\frac{-\,U}{2}\]
You need to login to perform this action.
You will be redirected in
3 sec
