| Define the term work function of a metal. The threshold frequency of a metal is \[{{f}_{0}}.\] When the light of frequency \[2{{f}_{0}}\] is incident on the metal plate, the maximum velocity of electrons emitted is \[{{K}_{1}}.\] When the frequency of the incident radiation is increased to \[5{{f}_{0}},\] the maximum velocity of electrons emitted is \[{{k}_{2}}.\] Find the ratio of \[{{V}_{1}}\] to \[{{V}_{2}}.\] |
| Or |
| The following graph shows the variation of stopping potential \[{{V}_{s}}\]with the frequency (v) of the incident radiation for two photo sensitive metals X and Y. |
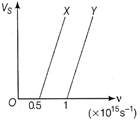 Variation of stopping potential for two
Variation of stopping potential for two | (i) Which of the metals has larger threshold wavelength? Give reasons. |
| (ii) Explain giving reason which metal gives out electrons having larger kinetic energy for the same wavelength of incident radiation. |
| (iii) If the distance between the light source and metal X is halved, then what will be the kinetic energy of electron emitted due to this change? Give reasons. |
Answer:
\[\frac{1}{2}\] Work function \[({{\phi }_{0}})\]A minimum amount of energy required to just eject an electron from the target metal is known as work function of the metal. The maximum kinetic energy of electrons emitted in a photoelectric effect, \[{{K}_{\max }}=hf-{{\phi }_{0}}\] Where, \[{{\phi }_{0}}={{f}_{0}}h\] is called the work function. \[{{K}_{1}}=h\times 2{{f}_{0}}-h{{f}_{0}}\] \[{{K}_{2}}=h\times 5{{f}_{0}}-h{{f}_{0}}=4h{{f}_{0}}\] \[\therefore \] \[\frac{{{K}_{1}}}{{{K}_{2}}}=\frac{1}{4}i.e\frac{\frac{1}{2}mv_{1}^{2}}{\frac{1}{2}mv_{2}^{2}}=\frac{1}{4}\] \[\therefore \] \[\frac{{{v}_{1}}}{{{v}_{2}}}=\frac{1}{2}\] Or (i) Threshold wavelength \[{{\lambda }_{0}}=\frac{c}{{{v}_{0}}}\] From the given graph, \[{{v}_{0}}(Y)>{{v}_{0}}(X)\] \[\therefore \] \[{{\lambda }_{0}}(Y)<{{\lambda }_{0}}(X)\] \[\therefore \] The metal X has larger threshold wavelength. (ii) \[hv=h{{v}_{0}}+KE\] of photoelectron or \[hc\,/\,{{\lambda }_{0}}+KE\] of photoelectron \[\therefore \] KE of photoelectron\[=h\lambda -h{{\lambda }_{0}}\] If \[\lambda \] is constant, then the metal having bigger \[{{\lambda }_{0}}\] gives out electrons having larger KE, i.e. metal X. (iii) The kinetic energy of electrons emitted does not depend on the distance between the light source and the metal.
You need to login to perform this action.
You will be redirected in
3 sec
