Answer:
Part A
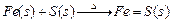
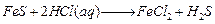 (1)
Part B
(1)
Part B
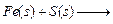 Mixture of iron filings and sulphur on adding
Mixture of iron filings and sulphur on adding 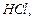
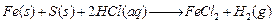 Sulphur remains unreacted. (1)
Identification
Sulphur remains unreacted. (1)
Identification
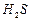 gas formed has a foul smell and on passing through lead acetate solution, it turns the solution black. (2)
Hydrogen gas burns with a pop sound.
OR
(i) Number as mass per cent
gas formed has a foul smell and on passing through lead acetate solution, it turns the solution black. (2)
Hydrogen gas burns with a pop sound.
OR
(i) Number as mass per cent
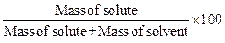 (1)
(ii) Meena prepared solution of concentration.
(1)
(ii) Meena prepared solution of concentration.
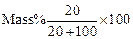 (2)
(2)
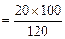
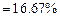 (2)
Rita prepared solution of concentration.
(2)
Rita prepared solution of concentration.
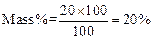 So, the solution prepared by Rita has higher mass percentage than that of prepared by Meena. (2)
So, the solution prepared by Rita has higher mass percentage than that of prepared by Meena. (2)
You need to login to perform this action.
You will be redirected in
3 sec
