
Answer:
A mixture of kerosene and petrol
(b.p. differ by more than 25oC) can be separated by
the process of simple distillation. (1)
Method
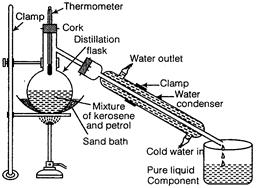
In a distillation flask, a mixture of kerosene and petrol is taken as shown in
figure. The mixture is heated slowly and the temperature is noted with the help
of thermometer. Petrol (b.p. = 70°C to 120°C) vaporizes first and the
temperature becomes constant for some time (till all petrol evaporates from the
mixture).
Vapours
of petrol are condensed and collected in another container, while the kerosene
remains in the distillation flask. Again the temperature starts rising and the
heating is stopped and both the components are collected separately.
This
is shown in the diagram below (2)
 (2)
Or
(i)
(a) Benzoic acid begin to melt at 20 min. (1)
(b)
Benzoic acid begin to boil at 52 min. (1)
(ii)
The melting point of benzoic acid is 120° C (1)
(iii)
The temperature remains constant at 120°C un till all the benzoic acid has
melted. (1)
(iv)
The physical state of benzoic acid is liquidduring the time interval of 35-45
min. (1)
(2)
Or
(i)
(a) Benzoic acid begin to melt at 20 min. (1)
(b)
Benzoic acid begin to boil at 52 min. (1)
(ii)
The melting point of benzoic acid is 120° C (1)
(iii)
The temperature remains constant at 120°C un till all the benzoic acid has
melted. (1)
(iv)
The physical state of benzoic acid is liquidduring the time interval of 35-45
min. (1)
You need to login to perform this action.
You will be redirected in
3 sec
