A) 1 atm
B) 4/5 atm
C) 4/3 atm
D) 3/2 atm
Correct Answer: C
Solution :
| [c] Initial pressure, \[{{p}_{1}}={{p}_{2}}={{p}_{0}}=\frac{nR{{T}_{0}}}{{{V}_{0}}}\] when |
| \[{{p}_{0}}=1\] atm \[{{T}_{0}}=300K.\] |
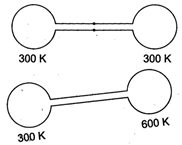 |
| Finally, let common pressure is p. |
| So, \[\frac{p{{V}_{0}}}{R\times 2{{T}_{0}}}={{n}_{1}}\] and \[\frac{p{{V}_{0}}}{R{{T}_{0}}}={{n}_{2}}\] and \[{{n}_{1}}+{{n}_{2}}=2n\] |
| \[\frac{p{{V}_{0}}}{2R{{T}_{0}}}+\frac{p{{V}_{0}}}{R{{T}_{0}}}=2\left( \frac{{{p}_{0}}{{V}_{0}}}{R{{T}_{0}}} \right)\] |
| \[\Rightarrow \] \[p=\frac{4}{3}{{p}_{0}}=\frac{4}{3}atm\] |
You need to login to perform this action.
You will be redirected in
3 sec
