A) \[a,\,\sqrt{2}a,\sqrt{3}a\]
B) \[\sqrt{3}a,\,\sqrt{2}a,\,a\]
C) \[a,\,\sqrt{2}a,2\,a\]
D) \[a,\,\sqrt{3}a,2\,a\]
Correct Answer: A
Solution :
[a] 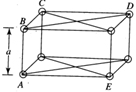 |
| \[AB=a\] (Nearest) |
| \[BD=\sqrt{2}a\] (Next-nearest) |
| \[CE=\sqrt{3}a\] (Next-next-nearest) |
You need to login to perform this action.
You will be redirected in
3 sec
