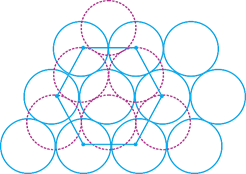
A) In one unit cell there are 12 octahedral voids and all are completely inside the unit cell.
B) In one unit cell there are six octahedral voids and all are completely inside the unit cell.
C) In one unit cell there are six octahedral void and of which three are completely inside the unit cell and other three are partially inside the unit cell.
D) In one unit cell there are 12 tetrahedral voids, all are completely inside the unit cell.
Correct Answer: B
Solution :
[b] HCP = AB AB AB pattern repeat For calculating voids between two layers A and B. Total tetrahedral voids =12 (represented by cross). All are completely inside.You need to login to perform this action.
You will be redirected in
3 sec
