A) \[{{X}_{A}}=0.30,{{X}_{B}}=0.70\]
B) \[{{X}_{A}}=0.40,{{X}_{B}}=0.60\]
C) \[{{X}_{A}}=0.70,{{X}_{B}}=0.30\]
D) \[{{X}_{A}}=0.50,{{X}_{B}}=0.50\]
Correct Answer: C
Solution :
| [c] \[p_{A}^{0}=1000\] torr \[p_{B}^{0}=1600\] |
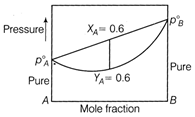 |
| \[{{X}_{A}}=0.6\] \[{{Y}_{A}}=0.6\] (from graph) |
| \[{{Y}_{A}}=\frac{p_{A}^{0}{{X}_{A}}}{p_{A}^{0}{{X}_{A}}+p_{B}^{0}(1-{{X}_{A}})}\] |
| \[0.6=\frac{1000{{X}_{A}}}{1000{{X}_{A}}+1600(1-{{X}_{A}})}\] |
| \[X{{X}_{A}}=0.70\,\,X{{X}_{B}}=1-{{X}_{A}}=0.30\] |
You need to login to perform this action.
You will be redirected in
3 sec
