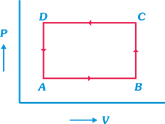
A) \[\Delta U(C\to D)=\] negative
B) \[\Delta Q(A\to B)=\] positive
C) \[\Delta U=(A-B-C-D-A)\ne 0\]
D) \[\Delta Q(D\to A)=0\]
Correct Answer: D
Solution :
[d] Idea In an isolated system no heat is exchanged between system and surrounding during process. Here as system is isolated \[\Delta Q=0,\] for any part of system in graph. TEST Edge Question from p-V curve are very important from examination point of view, conceptual clearity is necessary to tackle. These problems, a problem which seem difficult may involve a basic concept with which it can be solved easily. Work done in p-V curve, change in internal energy etc., are common questions which are asked in examination.You need to login to perform this action.
You will be redirected in
3 sec
