A) electrons
B) neutrons
C) nucleus
D) protons
Correct Answer: C
Solution :
Key Idea: In the experiment a -particles are deflected, at small angles. In Rutherford's experiment a fine beam of high energy\[\alpha -\]particles emitted by radioactive element was made to fall on a very thin gold foil.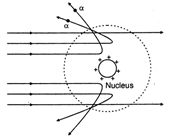 Some \[\alpha -\]particles are deflected at small angles and their angular distribution is definite. Now since \[\alpha -\]particles are positively charged, the part of the atom deflecting them must also be positive. On this basis Rutherford concluded that the whole of the positive charge of atom must be concentrated in a very small space. Thus, nucleus was discovered. Electrons revolve around the nucleus.
Some \[\alpha -\]particles are deflected at small angles and their angular distribution is definite. Now since \[\alpha -\]particles are positively charged, the part of the atom deflecting them must also be positive. On this basis Rutherford concluded that the whole of the positive charge of atom must be concentrated in a very small space. Thus, nucleus was discovered. Electrons revolve around the nucleus.
You need to login to perform this action.
You will be redirected in
3 sec
