A) \[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
B) \[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
C) \[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,H{{C}_{6}}{{H}_{5}}\]
D) \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
Correct Answer: C
Solution :
Carbonium ions \[({{C}^{+}})\] are electron deficient species with sextet of electron. The order of stability of carbonium ion can be explained on the basis of inductive effect. Alkyl group are electron rich or electron donors and bigger is the alkyl group more is its electron donating tendency (\[+I\] effect)
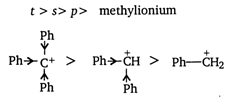 The most stable carbonium ion is \[{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{+}{\mathop{C}}\,H.\]
The most stable carbonium ion is \[{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{+}{\mathop{C}}\,H.\]
You need to login to perform this action.
You will be redirected in
3 sec
