A) \[{{B}_{6}}{{H}_{6}}\]
B) \[{{B}_{5}}N{{H}_{6}}\]
C) \[{{B}_{4}}{{N}_{2}}{{H}_{6}}\]
D) \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\]
Correct Answer: D
Solution :
Borazine \[({{B}_{3}}{{N}_{3}}{{H}_{6}})\] is also known as inorganic benzene and has structure similar to that of benzene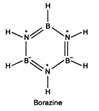 It is prepared as follows \[2{{B}_{2}}{{H}_{6}}+6N{{H}_{3}}\xrightarrow{450\,K}2{{B}_{3}}{{N}_{3}}{{H}_{6}}+12{{H}_{2}}\uparrow \]
It is prepared as follows \[2{{B}_{2}}{{H}_{6}}+6N{{H}_{3}}\xrightarrow{450\,K}2{{B}_{3}}{{N}_{3}}{{H}_{6}}+12{{H}_{2}}\uparrow \]
You need to login to perform this action.
You will be redirected in
3 sec
