A) \[FeC{{l}_{3}}\]
B) \[FeB{{r}_{2}}\]
C) \[AlC{{l}_{3}}\]
D) \[NaCl\]
Correct Answer: D
Solution :
Friedel-Craft's reaction: This reaction is used for alkylation or acylation of benzene and is initiated by electrophilic attack on benzene. Electrophile is produced in the presence of any Lewis acid \[viz\,AlC{{l}_{3}}\,FeC{{l}_{3}},\,FeB{{r}_{3}}\]etc. \[\text{NaCl}\]is not a Lewis acid hence, cannot be used.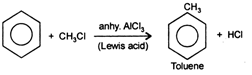
 Electrohile is generated as follows \[C{{H}_{3}}Cl+AlC{{l}_{3}}\xrightarrow{{}}AlCl_{4}^{-}+\underset{\begin{smallmatrix} (Carbocation\, \\ E{{l}^{+}}) \end{smallmatrix}}{\mathop{^{+}C{{H}_{3}}}}\,\]
Electrohile is generated as follows \[C{{H}_{3}}Cl+AlC{{l}_{3}}\xrightarrow{{}}AlCl_{4}^{-}+\underset{\begin{smallmatrix} (Carbocation\, \\ E{{l}^{+}}) \end{smallmatrix}}{\mathop{^{+}C{{H}_{3}}}}\,\] 

You need to login to perform this action.
You will be redirected in
3 sec
