A) adiabatic
B) isobaric
C) isothermal
D) equal in all above cases
Correct Answer: B
Solution :
The \[P-V\] diagram for isobaric, isothermal and adiabatic process of an ideal gas is shown in graph below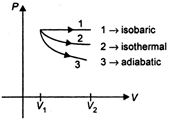 In thermodynamics, for same change in volume, the work done is maximum for the curve having maximum area enclosed with the volume axis. Area enclosed by the curve \[\propto \] (Slope of curve) As shown \[{{(slope)}_{isobaric}}<{{(slope)}_{isothermal}}<{{(slope)}_{adiabatic}}\] \[\Rightarrow \] \[{{(Area)}_{isobaric}}>{{(Area)}_{isorthermal}}>{{(Area)}_{adiabatic}}\] Hence, work done is maximum in isobaric process.
In thermodynamics, for same change in volume, the work done is maximum for the curve having maximum area enclosed with the volume axis. Area enclosed by the curve \[\propto \] (Slope of curve) As shown \[{{(slope)}_{isobaric}}<{{(slope)}_{isothermal}}<{{(slope)}_{adiabatic}}\] \[\Rightarrow \] \[{{(Area)}_{isobaric}}>{{(Area)}_{isorthermal}}>{{(Area)}_{adiabatic}}\] Hence, work done is maximum in isobaric process.
You need to login to perform this action.
You will be redirected in
3 sec
