A) \[t\,\,vs\,\,\log \,\,{{C}_{0}}\]
B) \[t\,\,vs\,\,\log \,{{C}_{t}}\]
C) \[{{t}^{-1}}\,\,vs\,\,\log \,{{C}_{t}}\]
D) \[\log \,\,{{C}_{t}}\,vs\,\,\log \,\,{{C}_{t}}\]
Correct Answer: B
Solution :
\[K\,\,f=-\log {{C}_{0}}-\log {{C}_{t}}\] \[y=mx+C\] \[\log {{C}_{t}}=\frac{-K\,t}{2.303}+\log {{C}_{0}}\]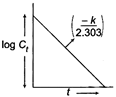
You need to login to perform this action.
You will be redirected in
3 sec
