A)
![]()
B)
![]()
C)
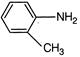
D)
![]()
Correct Answer: D
Solution :
The basicity of amine group depends on the lone pair present on N atom. If this lone pair involved in the conjugation, the basic character of amine decreases. So, find the molecule in which lone pair of nitrogen does not involve in conjugation.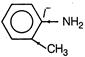
You need to login to perform this action.
You will be redirected in
3 sec
