 AIEEE Solved Paper-2005
AIEEE Solved Paper-2005
A) (C), (B), (A), (D)
B) (B), (C), (A), (D)
C) (D), (C), (B), (A)
D) (A), (B), (C), (D)
Correct Answer: B
Solution :
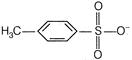
If acid is weak, its conjugate base (nucleophile) is strong and vice-versa. \[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ |\,| \end{smallmatrix}}{\mathop{C}}\,-{{O}^{-}}\]is a conjugate base of \[\overset{\begin{smallmatrix} O \\ |\,| \end{smallmatrix}}{\mathop{C{{H}_{3}}COH}}\,(I)\] \[C{{H}_{3}}{{O}^{-}}\]is a conjugate base of\[C{{H}_{3}}OH\](II) \[C{{N}^{-}}\]is a conjugate base of\[HCN\](III)You need to login to perform this action.
You will be redirected in
3 sec
