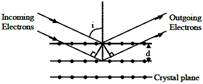
 If a strong diffraction peak is observed when electrons are incident at an angle 'I' from the normal to the crystal planes with distance 'd' between them (see figure), de Broglie wavelength \[{{\lambda }_{dB}}\] of electrons can be calculated by the relationship (n is an integer)
AIEEE Solved Paper-2007
If a strong diffraction peak is observed when electrons are incident at an angle 'I' from the normal to the crystal planes with distance 'd' between them (see figure), de Broglie wavelength \[{{\lambda }_{dB}}\] of electrons can be calculated by the relationship (n is an integer)
AIEEE Solved Paper-2007
A) \[2\,d\sin i=n{{\lambda }_{dB}}\]
B) \[d\cos i=n{{\lambda }_{dB}}\]
C) \[d\sin i=n{{\lambda }_{dB}}\]
D) \[2d\cos i=n{{\lambda }_{dB}}\]
Correct Answer: D
Solution :
For strong peak, path difference \[=2n{{\lambda }_{dB}}\] \[\therefore \,\,\,2d\,\cos i=n\lambda {{d}_{dB}}\]You need to login to perform this action.
You will be redirected in
3 sec
