A) 3-nitrochlorobenzene
B) 1 -nitrochlorobenzene
C) 4-nitrochlorobenzene
D) none of these.
Correct Answer: A
Solution :
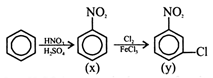 \[(HN{{O}_{3}}+{{H}_{2}}S{{O}_{4}})\] reagent is the agent for nitration in aromatic rings. \[HN{{O}_{3}}+2{{H}_{2}}S{{O}_{4}}\to NO_{2}^{+}+HSO_{4}^{-}+{{H}_{3}}{{O}^{+}}\]
\[(HN{{O}_{3}}+{{H}_{2}}S{{O}_{4}})\] reagent is the agent for nitration in aromatic rings. \[HN{{O}_{3}}+2{{H}_{2}}S{{O}_{4}}\to NO_{2}^{+}+HSO_{4}^{-}+{{H}_{3}}{{O}^{+}}\]  \[N{{O}_{2}}\] group is a meta directing group so chlorine atom goes to meta position of the ring.
\[N{{O}_{2}}\] group is a meta directing group so chlorine atom goes to meta position of the ring.
You need to login to perform this action.
You will be redirected in
3 sec
