A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) linear
Correct Answer: C
Solution :
\[N{{H}_{3}}\] is \[s{{p}^{3}}\] hybridised with trigonal pyramidal structure. N atom of \[N{{H}_{3}}\] has a lone pair electron which distorts its geometry due to \[lp-bp\] interaction to reduce the bond angle to \[{{107}^{o}}\].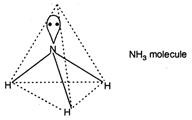
You need to login to perform this action.
You will be redirected in
3 sec
